Nanomedicine innovations
Revolutionizing therapeutic delivery for personalized medicine.

Extracellular vesicles and synthetic lipid nanoparticles are hybridized and functionalized to create a hybrid nanoparticle for specific and safe therapeutic in vivo delivery.
Exosomes are naturally occurring extracellular vesicles, that serve as biocompatible and cell-specific delivery vehicles, capable of carrying genetic material and proteins while evading immune detection.
A controlled loading methodology of fusion between EVs and mRNA-loaded liponanoparticles enables a single-particle quality level.
Liponanopartcles (LNP) are widely used as drug delivery vesicles, but present several drawbacks like low biocompatibility and unfavorable immune response.
What are Exo-LNPs?
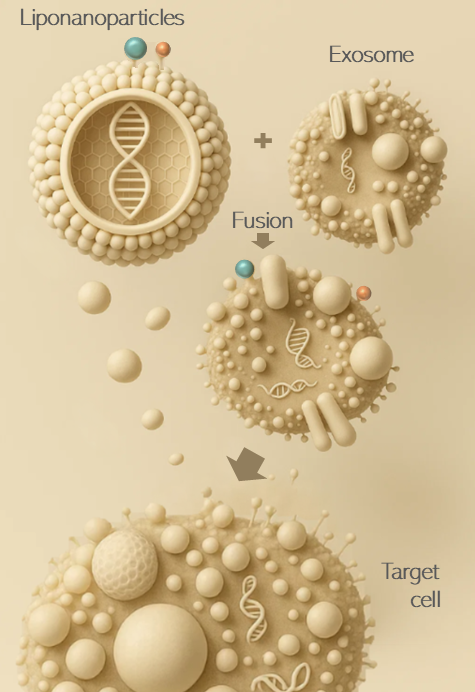
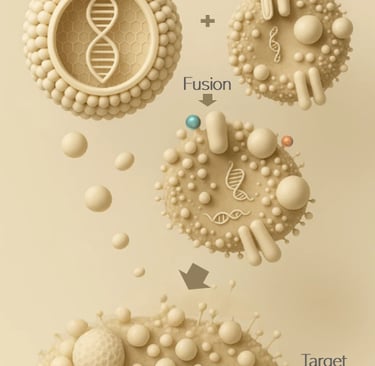
Exosome–LNP hybrids enable in vivo delivery of active immunotherapies by transferring the payload directly to the targeted cell.


What are CAR-T cells?
CAR-T cells are a type of immune cell that has been genetically engineered to recognize and kill cancer cells using a synthetic receptor called a chimeric antigen receptor (CAR).
CAR-T cells technology present major drawbacks
Although a major success in leukemia, current CAR-T therapy has shown frequent relapses and no second infusion is allowed. Besides, it presents a very high cost of manufacturing and efficacy in solid tumor has not been demonstrated.
CAR-T cells therapy require major Unlock-keys: safer, faster scalable and next generation technology platforms
CAR-T engineering
Regenerative Solutions
Current Delivery & Technology Challenges
Targeted delivery: Getting cells, biomaterials, growth factors, or extracellular vesicles to the right tissue in vivo without systemic side effects.
Controlled release: Sustained and localized release of therapeutic molecules remains hard to achieve, especially in dynamic tissues.
Biomaterial biocompatibility: Scaffolds and hydrogels need to avoid fibrosis, degradation issues, or chronic inflammation.
Scalability and reproducibility: Manufacturing stem cell products, exosomes, or engineered vectors under GMP conditions at scale is still expensive and technically complex.
Exo-LNP Impact versus “plain” EVs or LNPs
Compared with EVs: better nucleic-acid loading consistency and manufacturability; easier scale-up; more tunable composition.
Compared with LNPs: improved tissue access and potential cell-type tropism in dense ECM or immune-active sites, with possibly lower innate immune activation.
Exo-LNP Highlights
Deeper tissue penetration & tropism. Borrowing EV surface lipids/proteins or fully “exosome-inspired” shells can improve ECM penetration and homing versus conventional LNPs—important in fibrotic, ischemic, or avascular tissues.
Immune quieting & biocompatibility. EV components can reduce reactogenicity while retaining LNP-like NA loading/scale.
Versatile cargo. mRNA/miRNA/protein delivery relevant to regeneration (e.g., pro-angiogenic, anti-inflammatory, antifibrotic programs).
Future Directions
Neurological Disorders (Crossing the BBB)
Native exosomes cross the blood–brain barrier more efficiently than LNPs. Exo-LNPs could enhance delivery of:
mRNA for neurodegenerative diseases (Parkinson’s, ALS, Alzheimer’s).
Gene-editing payloads in rare CNS diseases.
Anti-inflammatory agents for multiple sclerosis or traumatic brain injury.
Infectious Diseases
mRNA vaccines: exo-LNPs may improve stability and reduce innate immune activation compared to classical LNPs.
Targeting tissues poorly reached by standard LNP vaccines (e.g. mucosal immunity for respiratory viruses, HIV reservoirs).
Delivery of CRISPR antivirals to infected cells.
Metabolic & Cardiovascular Diseases
Targeting adipose tissue, muscle, or myocardium — where classical LNP biodistribution is poor.
mRNA for angiogenesis post-MI.
siRNA for PCSK9 or other lipid regulators.
Modulating inflammation in atherosclerosis.








